Kolkata: In a significant breakthrough, researchers at the S. N. Bose National Centre for Basic Sciences, Kolkata, have developed an efficient, energy-saving, and environmentally friendly method to synthesize hydrogen peroxide (H₂O₂). This innovative approach offers a sustainable alternative to the traditional anthraquinone oxidation process, which is highly energy-intensive and generates hazardous by-products.
Hydrogen peroxide is a crucial chemical used in environmental disinfection, chemical synthesis, paper bleaching, and fuel cells. The demand for H₂O₂ has been steadily increasing due to rising awareness of hygiene, a growing number of surgical procedures, and concerns over hospital-acquired infections. However, current industrial production methods require high energy consumption and result in harmful waste.
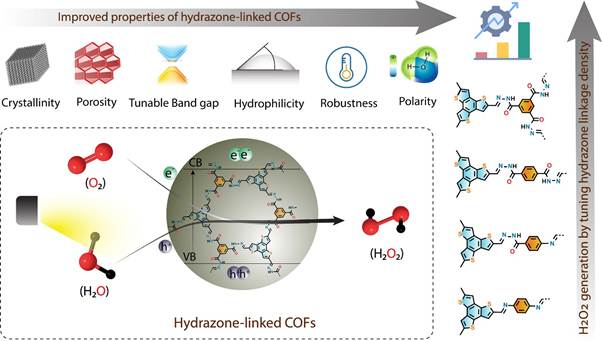
To address these challenges, scientists at the Department of Science and Technology (DST)-affiliated research center have explored a new class of materials known as covalent organic frameworks (COFs). These porous, ordered polymers have tunable catalytic sites and the ability to harness visible light, making them ideal photocatalysts for sustainable hydrogen peroxide synthesis.
By carefully modifying the hydrazone linkage density in COFs, the researchers were able to enhance water affinity, thereby improving the efficiency of the water oxidation reaction (WOR) and oxygen reduction reaction (ORR)—two key pathways for H₂O₂ generation. Under blue LED light irradiation (λ = 467 nm), the hydrazone-linked COF demonstrated exceptional photocatalytic performance without requiring external sacrificial electron donors. More notably, it achieved a significant hydrogen peroxide yield (550 μmol g⁻¹ h⁻¹) even under direct sunlight, surpassing most organic photocatalysts used under similar conditions.
Further advancements were made by utilizing a water-benzyl alcohol solution (90:10), which prevented the degradation of H₂O₂ and enabled production levels to reach an impressive 21,641 μmol g⁻¹ h⁻¹. This breakthrough suggests the potential for scaling up the technology into a continuous flow reactor, paving the way for industrial applications and widespread commercialization.
By demonstrating a cleaner, cost-effective, and scalable method for hydrogen peroxide production, the study marks a step forward in sustainable chemical manufacturing. This innovation has the potential to benefit multiple industries while significantly reducing environmental impact.