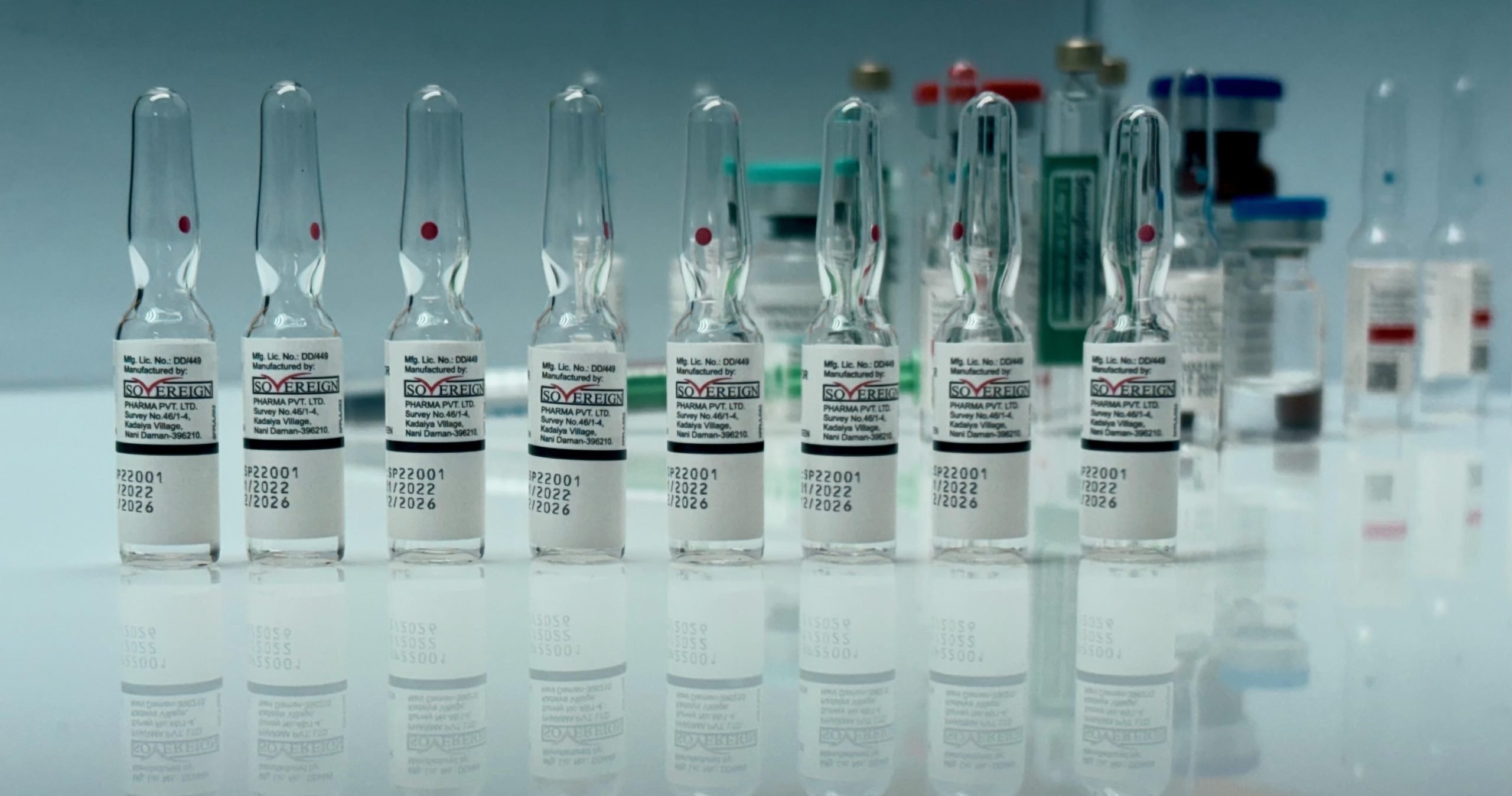
Mumbai: Sovereign Pharma has reached a major milestone with the European Union (EU) approval for its aseptic and terminally sterilized injectable products, including vials, ampoules, cartridges, and pre-filled syringes (PFS) for both liquid and lyophilized formulations. This achievement further solidifies the company’s commitment to global healthcare excellence and high manufacturing standards.
The EU certification follows approvals from ANVISA (Brazil) and MHRA (UK), all secured within the past year. These prestigious endorsements place Sovereign Pharma among the top global manufacturers of high-quality injectables, allowing the company to expand its reach into new international markets while maintaining stringent safety and quality protocols.
Founder Kairus Dadachanji emphasized the company’s unwavering commitment to quality, stating that every product manufactured at Sovereign Pharma adheres to the strictest safety, efficacy, and reliability standards. He added that the EU approval is a testament to the company’s manufacturing excellence and dedication to providing world-class pharmaceutical solutions.
With a well-established presence in over 50 countries, Sovereign Pharma is now poised for further expansion. The Brazilian Health Regulatory Agency (ANVISA) has approved aseptically processed and terminally sterilized small volume parenteral solutions, while the UK’s Medicines and Healthcare products Regulatory Agency (MHRA) has certified terminally sterilized small volume liquid vials. These approvals reinforce Sovereign Pharma’s reputation as a globally trusted manufacturer.
In a move to further strengthen its position, Sovereign Pharma is investing €30 million to enhance its manufacturing capabilities. The investment will support the integration of isolator filling lines, automated lyophilization systems, fully automated packing lines, and the development of a dedicated facility for pre-filled syringes and cartridge filling. The upgraded facility is expected to be operational by the end of 2025, aligning with the latest technological advancements and regulatory standards.
With this expansion, Sovereign Pharma continues to push the boundaries of sterile manufacturing, ensuring the delivery of safe, effective, and innovative injectable solutions to meet the growing demands of the global healthcare industry.
Bhubaneswar: With a renewed thrust on accelerating grassroots development, Chief Secretary Anu Garg held an…
Bhubaneswar: Odisha took a major stride towards building an industry-aligned and future-ready skilling ecosystem with…
Bhubaneswar: Marking a new chapter in Odisha’s public transport system, the Capital Region Urban Transport…
Bhubaneswar: With a focus on accelerating Odisha’s infrastructure growth, Works Minister Prithiviraj Harichandan on Tuesday…
Bhubaneswar: The Odisha Government has intensified its efforts to enhance the livelihood security, safety and…
Bhubaneswar: The long-awaited counting and inventory of gold ornaments and precious jewels housed in the…